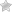A drug originally developed to prevent the rejection of transplanted organs has now been shown to dramatically reduce a particular kind of brain tumor in patients with tuberous sclerosis complex (TSC) — a genetic disease that causes tumors to grow on vital organs.
The study, published online in The Lancet, is the latest to show the effectiveness of everolimus in slowing the cell growth that is overactive in patients with TSC.
“Every patient in this study experienced a decrease in size of their tumors, and no patient required surgery for their tumors after treatment with everolimus,” says Dr. Franz, co-director of the TSC Clinic at Cincinnati Children’s and the study’s main author. “Thirty-five percent of patients in this study on everolimus had at least a 50 percent reduction in tumor volume after an average of 42 weeks on medication.”
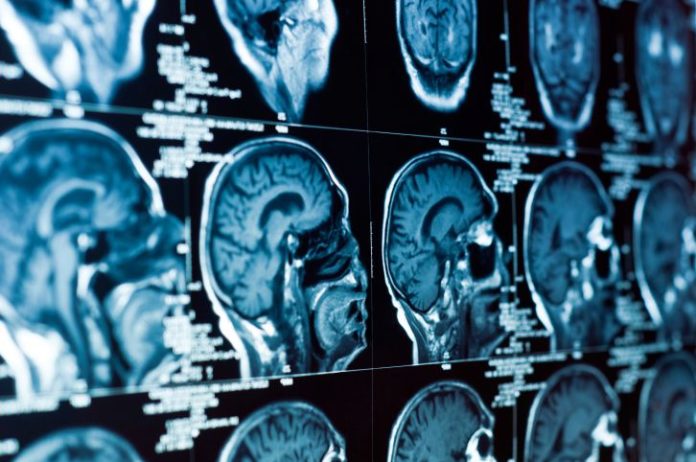
The phase III study was conducted among 117 patients with TSC who were randomly assigned to either everolimus or a placebo. Patients were 9 ½ years old on average but ranged from infants to adults. No patient on placebo showed improvement in their tumors. Tumor volume was measured by MRI assessment of the brain.
Dr. Franz conducted an earlier, phase II study of everolimus published in The New England Journal of Medicine in 2010. Based on that data, the U.S. Food and Drug Administration granted accelerated approval of everolimus for patients with these tumors, known as subependymal giant cell astrocytomas, or SEGAs. The new, placebo-controlled study was conducted to confirm these earlier results.
Prior to FDA approval, surgery was considered standard therapy for SEGAs, but everolimus is a potential alternative to surgery and the first targeted medical therapy for TSC, says Dr. Franz.
“Children and teens may not only avoid surgery but they also may see improvement in other aspects of this disease, including a reduction or even elimination of hydrocephalus – a buildup of fluid inside the skull leading to increased intracranial pressure. Hydrocephalus is commonly associated with these tumors because they are located deep within the brain in spinal fluid pathways, or ventricles.”
In Dr. Franz’s 2010 study, patients reported their quality of life, as measured by a validated quality of life and neuropsychological assessments, improved at three months and six months after treatment with everolimus.
The same mTOR pathway associated with overactive cell growth in TSC also is implicated in other cancers and neurological conditions, such as Alzheimer’s disease, type 2 diabetes, Parkinson’s disease, Huntington’s disease and autism. This makes everolimus, an mTOR inhibitor, a potential candidate to treat these mTOR-associated disorders, says Dr. Franz.
Based on studies by John Bissler, MD, a nephrologist at Cincinnati Children’s and co-director of the TSC clinic, the US Food and Drug Administration (FDA) earlier this year approved everolimus as the first medication to shrink non-cancerous kidney tumors that strike up to 80 percent of people with TSC. This allows many patients to maintain kidney function for years to come – without the need for repeated surgical intervention.
The TSC Clinic at Cincinnati Children’s is believed to be the largest in the world. The multidisciplinary clinic team follows more than 800 children and adults with tuberous sclerosis and manages every aspect of the disorder. The TSC Clinic recently celebrates its 20th anniversary.
Nearly 50,000 children and adults in the United States and approximately a million people around the world live with TSC.
Everolimus is marketed by Novartis, which provided drug and financial support for the study. In addition, several of the study authors are employees of Novartis.
Source: Cincinnati Children’s