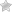Consumers and health professionals are advised that Kadac, in consultation with the TGA, is recalling all batches of Hyland’s Baby teething tablets and Hyland’s Baby nighttime teething tablets.
Hyland’s homoeopathic teething tablets are complementary medicines marketed to provide temporary relief of teething symptoms in children.
This recall is being undertaken following an investigation by the United States Food and Drug Administration that products supplied in the US contained inconsistent amounts of belladonna alkaloids, a toxic substance, which may differ from the calculated amounts listed on the products’ labels.
While the TGA had tested samples of these products supplied in Australia and found no quality issues, Kadac is now recalling the tablets as a precautionary measure due to the potential safety risk that belladonna alkaloids can pose to children. The effects of belladonna can be unpredictable and could cause serious health problems.
Please note that the Hyland’s Baby teething gel is not affected by this recall. However, all Hyland’s baby teething products, including the gel, will no longer be marketed in Australia.
Certain homoeopathic products, including the Hyland’s baby teething products, are not required to be on the Australian Register of Therapeutic Goods and are not assessed by the TGA prior to their entry into the Australian marketplace. However, this does not exclude them from other provisions of the Therapeutic Goods Act 1989 in relation to quality and safety standards.
Information for consumers
If you have any Hyland’s Baby teething tablets or Hyland’s Baby nighttime teething tablets, do not use them. Return them to the place of purchase for a refund or call Kadac on 1300 762 025.
If you have any questions or concerns about this issue, talk to your health professional.
Information for health professionals
Please be aware of this issue and advise patients accordingly if they seek advice.
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to the TGA’s monitoring of these products.
The TGA cannot give advice about an individual’s medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.
(Source: Australian Government – Therapeutic Goods Administration)